Pyrite, commonly known as “Fool’s Gold,” is one of the most fascinating and widely recognized minerals. With its metallic luster and gold-like appearance, it has been mistaken for real gold by prospectors for centuries. However, pyrite is far from worthless—it has significant industrial applications, a rich history, and unique properties that make it a valuable mineral in its own right.
In this article, we’ll explore the origins, properties, uses, and famous locations of pyrite, as well as why this mineral continues to be highly sought after today.
Table of contents / Jump to
What is Pyrite?
How Does Pyrite Form?
Physical and Chemical Properties of Pyrite
Pyrite vs. Gold: Key Differences
Industrial and Commercial Uses of Pyrite
Famous Pyrite Locations
The Role of Pyrite in Scientific Research
Conclusion
What is Pyrite?
Pyrite is a naturally occurring iron sulfide mineral with the chemical formula FeS₂. It has a striking brass-yellow color and a metallic luster, which is why it is often confused with gold. However, unlike gold, pyrite is brittle and will break rather than bend when pressure is applied.
Pyrite is one of the most common sulfide minerals and can be found in many different geological environments worldwide. It often forms in cubic or octahedral crystals and can sometimes occur in well-defined, shiny formations that make it highly collectible.
How Does Pyrite Form?
Pyrite forms in a variety of environments, ranging from sedimentary deposits to igneous and metamorphic rocks. Some of the primary ways pyrite is formed include:
- Sedimentary Rocks: Pyrite can develop in oxygen-poor environments, such as clay and shale formations, where iron and sulfur combine over time.
- Hydrothermal Veins: Pyrite often forms in association with gold, copper, and other valuable metals in hydrothermal deposits.
- Coal and Organic-Rich Environments: Pyrite can form in coal beds, where it interacts with decomposing organic material, sometimes leading to issues like acid mine drainage when exposed to water and oxygen.
- Volcanic Activity: Some pyrite deposits are formed as magma cools, crystallizing alongside other minerals in volcanic rocks.
Physical and Chemical Properties of Pyrite
| Property | Description |
| Chemical Formula | FeS₂ (Iron Sulfide) |
| Color | Pale brass-yellow, gold-like |
| Luster | Metallic |
| Hardness | 6 – 6.5 on the Mohs scale |
| Crystal System | Isometric (Cubic) |
| Density | 4.9 – 5.2 g/cm³ |
| Streak | Greenish-black to brownish-black |
| Fracture | Conchoidal to uneven |
A distinctive feature of pyrite is its ability to produce sparks when struck against metal. This property made it an important tool in early fire-starting techniques.
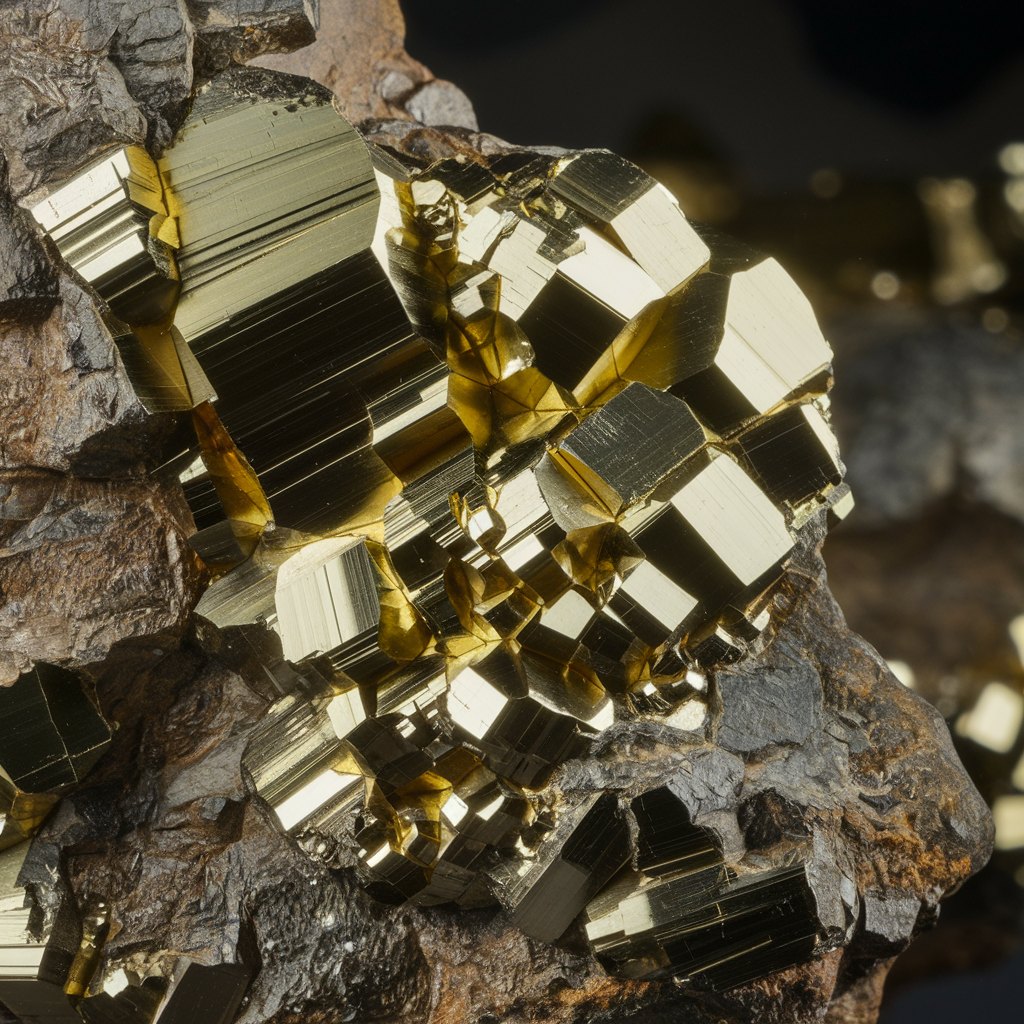
Pyrite vs. Gold: Key Differences
Because of its resemblance to gold, pyrite has historically fooled many prospectors. However, there are clear differences between the two minerals:
- Hardness: Pyrite is harder (6-6.5 on the Mohs scale), while gold is much softer (2.5-3).
- Malleability: Gold can be easily shaped and bent, while pyrite is brittle and breaks under pressure.
- Density: Gold is much denser (19.3 g/cm³) compared to pyrite (4.9-5.2 g/cm³).
- Streak Test: When rubbed against an unglazed tile, pyrite leaves a greenish-black streak, whereas gold leaves a yellow streak.
Industrial and Commercial Uses of Pyrite
Despite its nickname, pyrite has significant industrial and commercial value. Some of its main uses include:
1. Sulfur and Sulfuric Acid Production
Pyrite is one of the primary sources of sulfur, which is extracted and processed to produce sulfuric acid (H₂SO₄)—a key chemical used in:
- Fertilizers
- Chemical manufacturing
- Petroleum refining
- Battery production
2. Mining and Metal Extraction
Pyrite is often associated with gold, copper, and other valuable metals. In some cases, microscopic gold particles are embedded within pyrite, making it a secondary source of gold mining. Additionally, pyrite is sometimes processed to recover iron.
3. Fire-Starting and Early Tools
Before the invention of modern fire-starting tools, pyrite was used in combination with flint to create sparks for starting fires. This made it an essential tool for early civilizations.
4. Ore Processing and Smelting
Pyrite has been historically used as a source of iron in iron smelting. Although it is not as efficient as other iron ores, its widespread availability made it a useful resource in certain regions.
5. Fossil Preservation
One of the most fascinating natural roles of pyrite is in the preservation of fossils. Under specific conditions, pyrite can replace organic material in fossils, creating stunning pyritized fossils that are highly prized by collectors.
Famous Pyrite Locations
Pyrite is found all over the world, but some locations are particularly famous for producing high-quality specimens:
- Navajún, Spain: Known for its perfect cubic pyrite crystals, which are some of the most sought-after in the world.
- Peru: Produces beautiful pyrite formations, often found in association with quartz.
- United States: Pyrite is commonly found in states like Colorado, Pennsylvania, and Illinois. Some coal mines also contain significant pyrite deposits.
- China: One of the largest producers of pyrite for industrial use.
- Italy: The island of Elba is known for its stunning pyrite specimens.
The Role of Pyrite in Scientific Research
In addition to its industrial uses, pyrite has also gained interest in scientific research, particularly in fields like:
- Solar Energy: Scientists are studying pyrite for use in solar cells because of its semiconducting properties. If successfully developed, pyrite-based solar cells could provide an affordable alternative to traditional photovoltaic materials.
- Geological Studies: Pyrite is an important indicator mineral for geologists studying past environmental conditions and hydrothermal activity.
- Environmental Science: Pyrite plays a key role in acid mine drainage, a significant environmental issue where pyrite reacts with water and oxygen to form sulfuric acid, affecting water quality in mining regions.
Conclusion
While pyrite may not be real gold, it has a rich history, valuable industrial applications, and scientific significance. From its role in mining and sulfur production to its use in early fire-starting tools and fossil preservation, pyrite has proven to be more than just a trickster mineral.
If you’re looking for high-quality pyrite specimens, whether for collecting, scientific study, or decorative purposes, check out our mineral collection for authentic, hand-selected pieces.
Pyrite may have fooled many in the past, but today, we know its true value!





